Supine blood pressure was measured at each study visit1
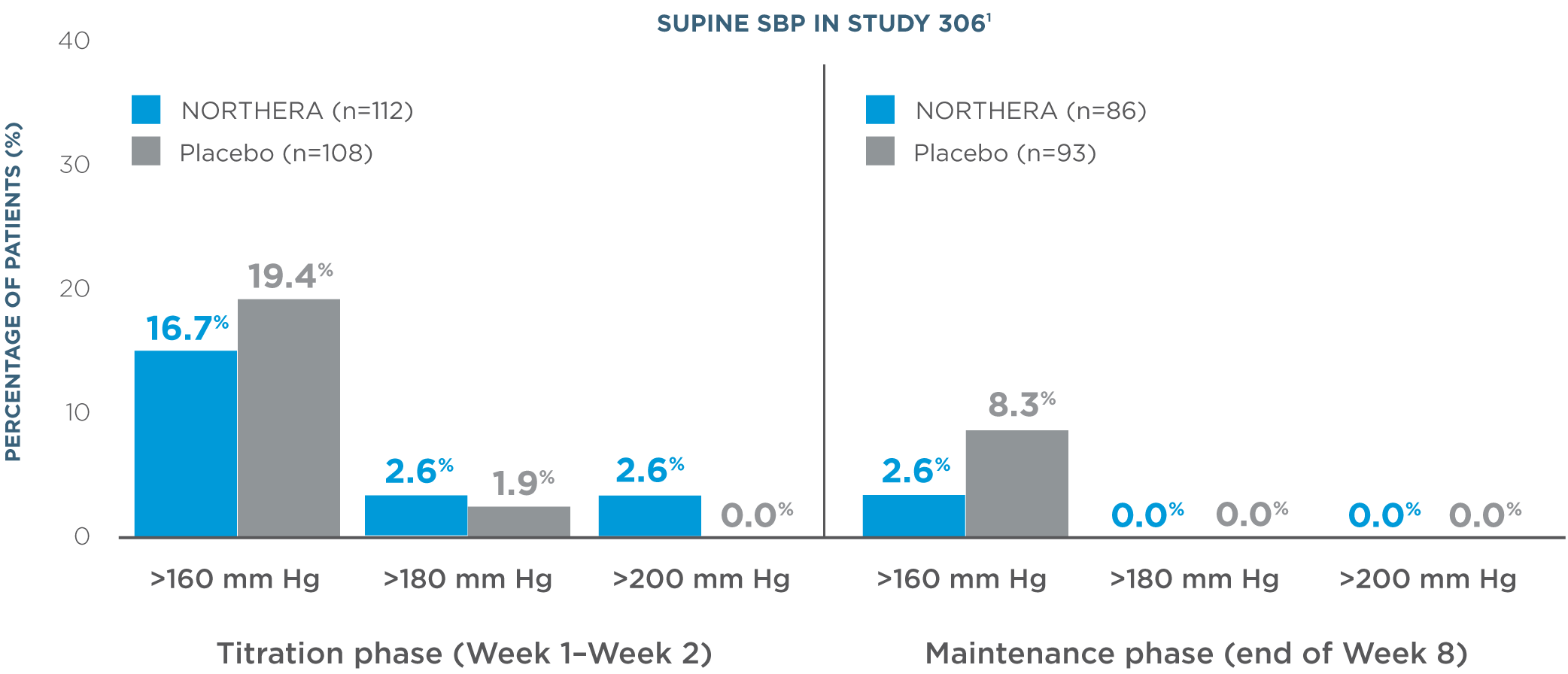
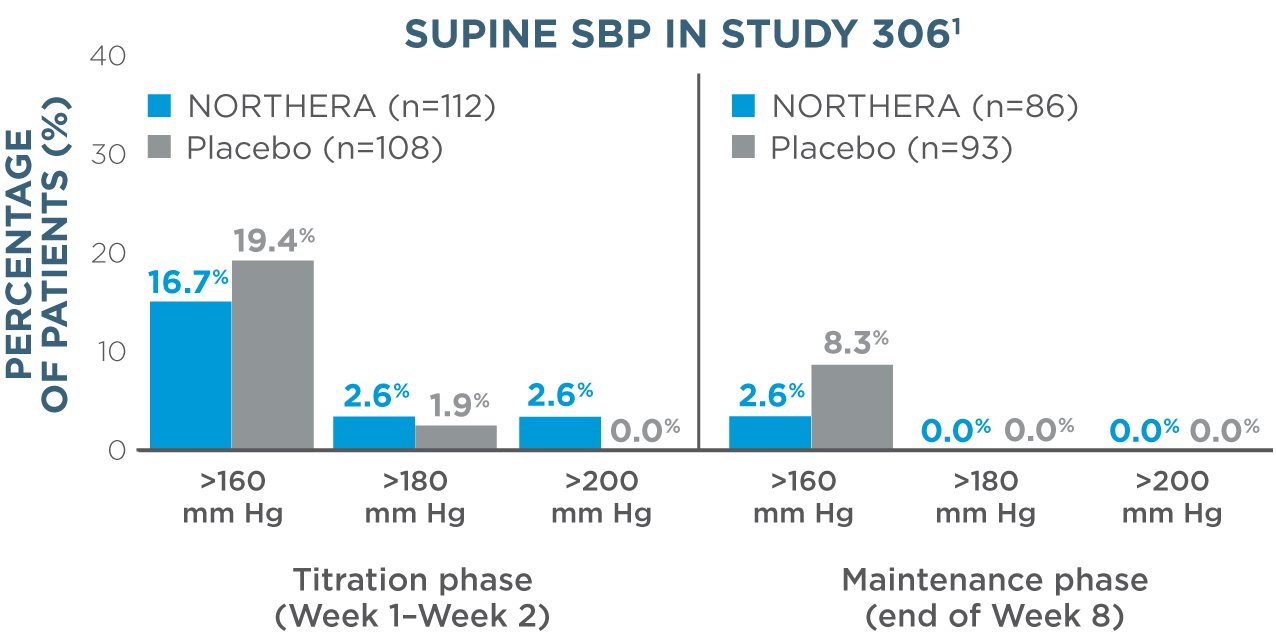
- Overall, rates of supine hypertension (SBP >180 mm Hg at all supine measurements during the orthostatic standing test) were slightly higher in NORTHERA® (droxidopa) patients (7.9%) compared with placebo patients (4.6%)1
- Supine blood pressure measurements were taken with upper body at 30-degree tilt 3 hours after administration of morning dose1
- Sustained severe hypertension (SBP ≥180 mm Hg or DBP ≥110 mm Hg in the seated or supine position observed in 3 consecutive measurements over an hour) at the screening visit was an exclusion criterion for the study1
- Dose escalation was stopped if supine SBP rose to ≥180 mm Hg or DBP to ≥110 mm Hg during dose escalation1
- To reduce the potential for supine hypertension, elevate the head of the bed and give the last dose of NORTHERA at least 3 hours prior to bedtime2
WARNINGS AND PRECAUTIONS
- Supine Hypertension: NORTHERA therapy may cause or exacerbate supine hypertension in patients with nOH, which may increase the risk of cardiovascular events if not well managed, particularly stroke.
- Hyperpyrexia and Confusion: Cases of a symptom complex resembling neuroleptic malignant syndrome (NMS) have been reported with NORTHERA use during post-marketing surveillance. Observe patients carefully when the dosage of NORTHERA is changed or when concomitant levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics. NMS is an uncommon but life-threatening syndrome characterized by fever or hyperthermia, muscle rigidity, involuntary movements, altered consciousness, and mental status changes. The early diagnosis of this condition is important for the appropriate management of these patients.
- Ischemic Heart Disease, Arrhythmias, and Congestive Heart Failure: NORTHERA therapy may exacerbate existing ischemic heart disease, arrhythmias, and congestive heart failure. Careful consideration should be given to this potential risk prior to initiating therapy.
- Allergic Reactions: Hypersensitivity reactions, including anaphylaxis, angioedema, bronchospasm, urticaria, and rash have been reported in post-marketing experience, with some resulting in emergency treatment. If a hypersensitivity reaction occurs, discontinue the drug and initiate appropriate therapy.
This product contains FD&C Yellow No. 5 (tartrazine), which may also cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
No prolongation of the QTc interval was observed in a QT study1,2
No prolongation of the QTc interval was observed with NORTHERA at single oral doses up to 2000 mg, as shown in a dedicated, thorough QT study in healthy volunteers.
Adverse reactions
Safety was assessed in controlled clinical trials and long-term, open-label studies
In the short-term, placebo-controlled studies, the most common adverse reactions were headache, dizziness, nausea, and hypertension2
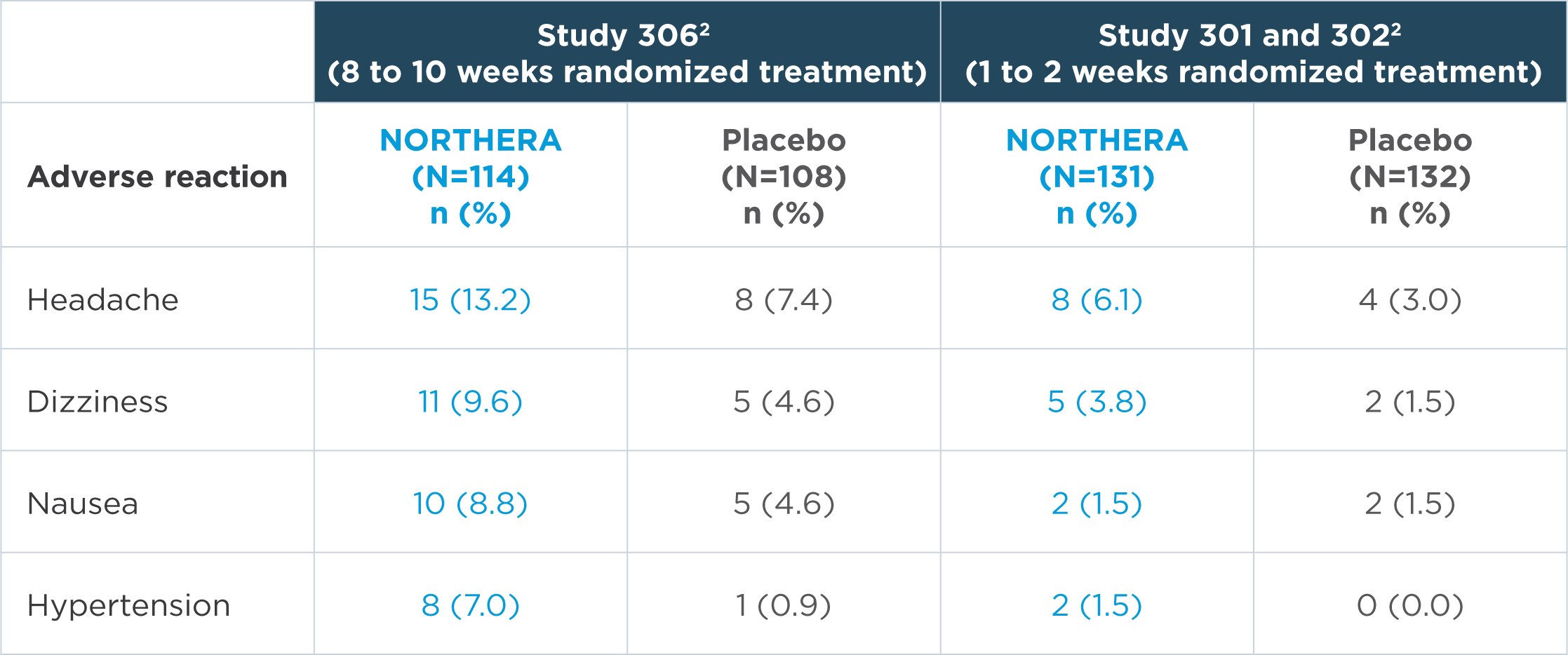
Note: n=number of patients. Adverse reactions that were reported in greater than 5% of patients in the NORTHERA group and with at least a 3% greater incidence in the NORTHERA group than in the placebo group were from Study 306.2
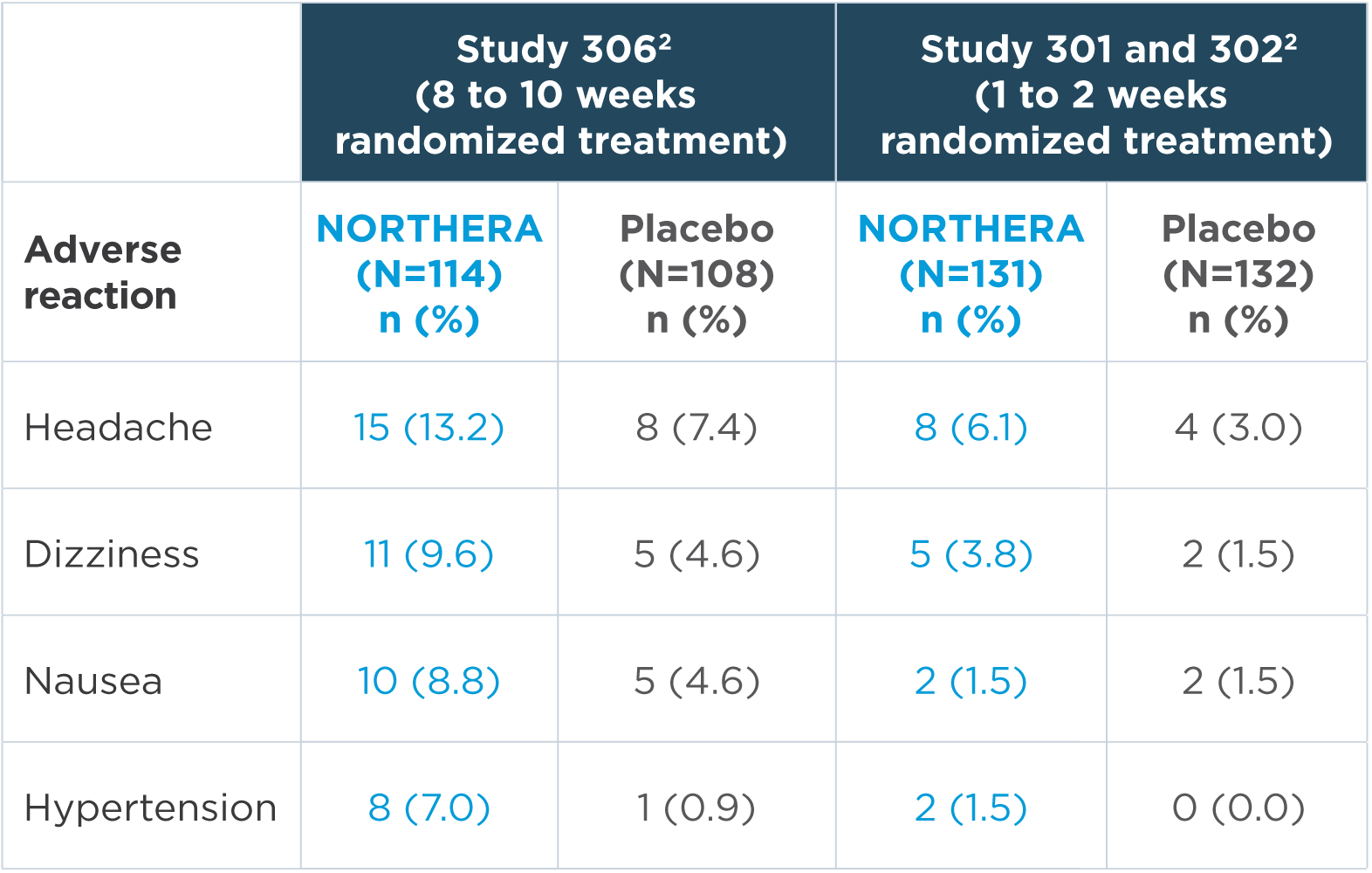
Note: n=number of patients. Adverse reactions that were reported in greater than 5% of patients in the NORTHERA group and with at least a 3% greater incidence in the NORTHERA group than in the placebo group were from Study 306.2
- The most common adverse reactions leading to discontinuation of NORTHERA were hypertension and nausea2
Long-term assessments of the safety of NORTHERA were conducted in both Study 303 and Study 3042
- Study 303 and Study 304 evaluated safety for 12 months and up to 36 months, respectively1,2
- In the long-term, open-label extension studies, a total of 422 patients, mean age 65 years, were treated with NORTHERA for a mean total exposure of approximately 1 year. The commonly reported adverse reactions were falls (24%), urinary tract infections (15%), headache (13%), syncope (13%), and dizziness (10%)2
References:
1. Data on file. Deerfield, IL: Lundbeck. 2. NORTHERA [package insert]. Deerfield, IL: Lundbeck.
Please see Important Safety Information, including Boxed Warning for supine hypertension.
For more information, see the full Prescribing Information.
Indications and Usage
NORTHERA (droxidopa) is indicated for the treatment of orthostatic dizziness, lightheadedness, or the “feeling that you are about to black out” in adult patients with symptomatic neurogenic orthostatic hypotension (nOH) caused by primary autonomic failure (Parkinson’s disease [PD], multiple system atrophy, and pure autonomic failure), dopamine beta-hydroxylase deficiency, and non-diabetic autonomic neuropathy. Effectiveness beyond 2 weeks of treatment has not been established. The continued effectiveness of NORTHERA should be assessed periodically.
Important Safety Information
WARNING: SUPINE HYPERTENSION
Monitor supine blood pressure prior to and during treatment and more frequently when increasing doses. Elevating the head of the bed lessens the risk of supine hypertension, and blood pressure should be measured in this position. If supine hypertension cannot be managed by elevation of the head of the bed, reduce or discontinue NORTHERA.
CONTRAINDICATIONS
- NORTHERA is contraindicated in patients who have a history of hypersensitivity to the drug or its ingredients.
WARNINGS AND PRECAUTIONS
- Supine Hypertension: NORTHERA therapy may cause or exacerbate supine hypertension in patients with nOH, which may increase the risk of cardiovascular events if not well managed, particularly stroke.
- Hyperpyrexia and Confusion: Cases of a symptom complex resembling neuroleptic malignant syndrome (NMS) have been reported with NORTHERA use during post-marketing surveillance. Observe patients carefully when the dosage of NORTHERA is changed or when concomitant levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics. NMS is an uncommon but life-threatening syndrome characterized by fever or hyperthermia, muscle rigidity, involuntary movements, altered consciousness, and mental status changes. The early diagnosis of this condition is important for the appropriate management of these patients.
- Ischemic Heart Disease, Arrhythmias, and Congestive Heart Failure: NORTHERA therapy may exacerbate existing ischemic heart disease, arrhythmias, and congestive heart failure. Careful consideration should be given to this potential risk prior to initiating therapy.
- Allergic Reactions: Hypersensitivity reactions, including anaphylaxis, angioedema, bronchospasm, urticaria, and rash have been reported in post-marketing experience, with some resulting in emergency treatment. If a hypersensitivity reaction occurs, discontinue the drug and initiate appropriate therapy.
- This product contains FD&C Yellow No. 5 (tartrazine), which may also cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
ADVERSE REACTIONS
- The most common adverse reactions (>5% and ≥3% difference compared to placebo) were headache, dizziness, nausea, and hypertension.
DRUG INTERACTIONS
- Administering NORTHERA in combination with other agents that increase blood pressure (e.g., norepinephrine, ephedrine, midodrine, and triptans) would be expected to increase the risk for supine hypertension.
- Dopa-decarboxylase inhibitors may require dose adjustments for NORTHERA.
- The concomitant use of selective MAO-B inhibitors, such as rasagiline or selegiline, was permitted in the NORTHERA clinical trials. However, based on mechanism of action, the use of non-selective MAO inhibitors and linezolid should be avoided as there is a potential for increased blood pressure when taken with NORTHERA.
USE IN SPECIFIC POPULATIONS
- There are no available data on use of NORTHERA in pregnant women and risk of major birth defects or miscarriage. Because of the potential for serious adverse reactions, including reduced weight gain in breastfed infants, advise a woman not to breastfeed during treatment with NORTHERA.
- The safety and effectiveness of NORTHERA in pediatric patients have not been established. No overall differences in safety or effectiveness were observed between patients aged 75 years and older and younger patients in clinical trials, but greater sensitivity of some older individuals cannot be ruled out.
- Clinical experience with NORTHERA in patients with severe renal function impairment (GFR <30 mL/min) is limited; therefore, dosing recommendations cannot be provided for these patients.
For more information, please see the full Prescribing Information, including Boxed Warning for supine hypertension.


